The ageing population is at risk of developing multiple chronic diseases leading to polypharmacy. Preventing harm from medications used in the elderly can be promoted by using explicit PIM criteria. Beer’s criteria, STOPP/START, and FORTA list were popular explicit criteria that were adopted into various criteria in Asian countries.

Higher life expectancy among Asian people leads to a high number of elderly aged 60 and over nowadays. This phenomenon is called the "ageing population,” where one of the negative impacts is higher costs in healthcare services. In 2002, the United Nations (UN) stated the "Madrid International Plan of Action on Ageing," which includes: i) reducing the cumulative effects of factors that increase the risk of disease and consequently potential dependence in older age; ii) developing policies to prevent ill health among older persons; and iii) to provide access to food and adequate nutrition for all older persons. The pan has been set for over 20 years, and countries have put various efforts in place to avoid healthcare risks for older people.
An epidemiological transition in all countries indicated a shift in the predominance of infectious diseases to chronic and degenerative diseases. The ageing population was at risk of developing chronic diseases such as diabetes, hypertension, obesity, and various mental health issues such as depression and dementia. Elderly or geriatric usually require age-related procedures and treatments that increase the cost of long-term care. This problem highlights the need for healthcare and education reforms to promote healthy ageing.
In promoting healthy ageing, the rational use of medicine is crucial. Rational use is defined as correct/ proper/ appropriate use of medications that refers to suitable selection, dose, duration, cost, and patients, and this medicine should be dispensed correctly and appropriately. Medicine against this principle will be considered irrational, incorrect, improper, or inappropriate.
Patient safety in the treatment process is one of the critical elements of qualified healthcare systems worldwide. Elderly patients undergo a series of pharmacological changes. The ability to maintain normal drug absorption was reduced in the elderly because of a rise in gastric pH, reduction in gastrointestinal motility and splanchnic blood flow. Drug distribution will also shift because of reduced lean body mass and increased body fat, which increase the distribution and prolong the half-life elimination of fat-soluble drugs. Metabolite clearance of hepatic and renal eliminated drugs usually decreases with age, slowing drug clearance and increasing some drugs' toxicity. In addition, the elderly also undergo pharmacodynamic changes because of the presence of medical illness and an increase in age. Many drugs are contraindicated for the elderly and require much caution.
Advanced age, along with multiple morbidity and polypharmacy, were predisposing factors for the occurrence of adverse effects. Multimorbidity in the elderly can impair drug handling and administration in chronic conditions. Multiple chronic conditions burdened in people with multimorbidity increase the risk of functional impairment, deterioration in quality of life, and increased mortality. Multimorbidity also increases the risk of polypharmacy caused by the coexistence of many diseases which require pharmacological treatments.
Inappropriate use of medications, especially in the elderly population, is well known by the term PIMs (Potentially Inappropriate Medications). The risk of PIM use increased as the number of drugs consumed increased. The presence of mental/behavioural disorders influences the increased risk of developing PIM besides polypharmacy.
Many policies were established to prevent the rational use of medicine; some policies that were essentially adopted by developed countries were policies to reduce costs and develop national guidelines. Standard treatment guidelines should be evidence-based and unbiased. Guidelines developed to avoid potentially inappropriate medications are introduced as explicit or PIM criteria in geriatric medicine. These criteria focus on PIMs among older people and are usually developed based on evidence from clinical trials, experts’ opinions, and Delphi methods. Explicit criteria differed from implicit PIM criteria, focusing primarily on the patient rather than drugs or disease. Implicit PIM criteria were timeconsuming and depended on the prescriber's knowledge and expertise. In contrast, explicit PIM criteria clearly defines which drugs cause PIMs in particular clinical circumstances.
Three popular explicit PIM criteria widely used worldwide are: Beers Criteria, STOPP/START Criteria, and FORTA list. Beer’s criteria were first published in 1991 and have undergone several updates. In 2011, the American Geriatric Society (AGS) took responsibility for updating and maintaining Beers Criteria. The recent update of Beers Criteria is the 2023 version. These criteria explain PIM into five categories: drugs to avoid in older adults, drugs to avoid in certain diseases or syndromes in older adults, drugs should be used with caution, drugs should be avoided or adjusted in older adults with renal disease, and critical drug-drug interactions to avoid in older adults.
The Screening Tool for Older Persons Potentially Inappropriate Prescriptions (STOPP) and the Screening Tool to Alert Doctors to the Right Treatment (START) were developed in 2008. The second version appeared in 2014, and the recent version was published in 2023. The STOPP criteria were organised according to the physiological system, and the START was designed to screen PIMs under-prescribing. In comparison, the FORTA (Fit for the Aged) list was introduced in 2008 and updated in 2015. This list is widely used across European countries based on data gathered from six regions. This list includes 264 medications/ classes according to diagnosis or clinical syndrome. FORTA list graded the drugs into four levels of expected clinical benefit for geriatric populations.
Most explicit PIM criteria were based on medications marketed in Europe or the United States, making the applicability of those criteria in Asian countries relatively low. Asian countries have regional variations in drug formularies and health systems that differ from Western countries. This limitation led to the development of various similar criteria across Asian countries, listed below:
1) Lists of Risk Drugs for Thai Elderly (LRDTE): developed from 2012 Beers Criteria and 2008 STOPP in Thailand. This list considered the age group in the elderly population and the severity of medication risk. This list also covered standard treatment guidelines and hospital formulary for Thai elderly.
2) Guidelines for the Safety of Pharmacotherapy in the Elderly (GL2015) and STOPP-Japan: GL2015 were developed in 2015 while STOPP-Japan in 2016, both directed to the elderly aged ≥75 years. The GL2015 contains a list of medications that require prudent administration and a list of medications that should be considered for administration. At the same time, STOPP-Japan uses the terms "drugs to be prescribed with caution" and "drug to consider starting" to explain PIM.
3) Japan FORTA: developed in 2020 using EURO-FORTA, OAC-FORTA (Oral Anticoagulants for Long-Term Treatment of Atrial Fibrillation in Older Patients), and LUTS-FORTA (Lower Urinary Tract Symptoms-FORTA) list followed by two Delphi rounds by 13 experts in geriatric pharmacotherapy. This list consists of 210 PIMs across 24 indications.
4) The Chinese Criteria: Criteria of potentially inappropriate medications for older adults in China published in 2017. This criterion includes medication risk and medication risk under a morbid state. According to the expert panel evaluation, the Chinese criteria were divided into high-risk and low-risk medications and categorised according to defined daily doses.
5) PIM-China: These criteria was developed in 2018 and categorises PIMs into general PIM and disease-specific PIMs.
6) PIM-Taiwan: developed in 2019 using pre-existing standards and categorises the PIM into General PIMs and Disease-specific PIMs.
7) STOPP-START Singapore NUH In-house Guidelines: developed in 2015 based on STOPP/START Criteria. This guideline includes 89 criteria consisting of 55 STOPP and 34 START criteria.
8) STOPP-Indonesia: This tool was developed in 2020 using STOPP/START as standard. STOPP-Indonesia consists of 81 PIM Criteria grouped into 13 sections based on organs/systems.
9) PIM-Korea: There are four versions of PIM-Korea: 2010, 2015, 2018, and 2022. Different researchers developed all of them. The 2022 version includes PIMs in general, potential drug interactions, disease-specific PIMs, dose adjustment and potential omissions.
10) PIM-Pakistan: developed in 2018 using Beers Criteria 2015 and STOPP/ START Criteria version 2. This list contains 32 PIMs categorised pharmacologically.
11) STOPP/START Sri Lanka: Sri Lanka's version of STOPP/START Criteria was developed in 2019 and includes 105 criteria.
12) PIM-Hong Kong: developed in 2021 using the PRISCUS list, STOPP version 2, and Beers 2015 Criteria. This list categorised PIMs into general and disease-specific PIMs.
Explicit PIM criteria simplify the medication optimisation process by alerting prescribers of potential irrational medicine use in specific circumstances. Some criteria have an RCT (Randomised Controlled Trial)-proven clinical benefit that increases patient-related outcomes. Intervention using explicit PIM criteria is proven to reduce adverse drug reactions, incidents of falls, medication costs, and PIMs. These criteria should be applied in routine medication review and prescribing practice for multimorbid older people, especially those who are exposed to polypharmacy.
Previous research involved various healthcare professionals in implementing explicit PIM criteria. Physicians, hospital or clinical pharmacists, geriatricians, nurses, physical therapists, psychologist dietitians, occupational therapists, and speech therapists participated in research about explicit PIM criteria implementation. Hospitals or clinical pharmacists conduct most studies. The application of explicit PIM criteria ranged from outpatients, hospitalised patients, and at-patient discharge.
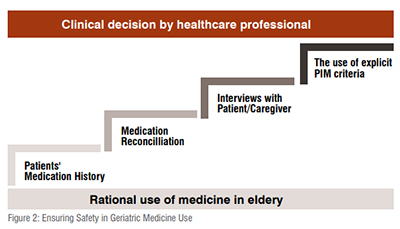
In order to avoid PIM using explicit PIM criteria, healthcare professionals need some reliable source of patient information that can support their clinical decisions. Besides medication history, healthcare professionals may need medication reconciliation, interviews with the patient or caregiver, and contact with pharmacists/ physicians. Reduction of PIM after intervention using explicit PIM criteria was found most successful through interprofessional collaboration by pharmacists and geriatricians. PIM use in older adults requiring long-term care should be reviewed from a multidimensional perspective.
Healthy ageing in the ageing population nowadays could be achieved by ensuring drug safety use in the elderly. Some components that affect the safety of drugs in geriatric patients are changes in the pharmacological and pharmacodynamics of drugs in the elderly. These changes lead to the occurrence of some potentially inappropriate medications if patients also consume polypharmacy due to multimorbidity. Rational use of medicine can be achieved by applying explicit PIM criteria in the prescribing process. Various explicit criteria were developed globally, and some Asian countries adopted some. This criterion has reduced PIMs in outpatients, hospitalised, and discharged elderly. Healthcare professionals must implement explicit PIM criteria in treating geriatric patients to avoid PIMs.